Meniscus Disease
If you have had a meniscus tear, you already know that the decision is between a partial removal or a repair, regeneration, or a replacement. How you make that decision is based, in part, on the health of the meniscus. Here are some updated insights into this tissue.
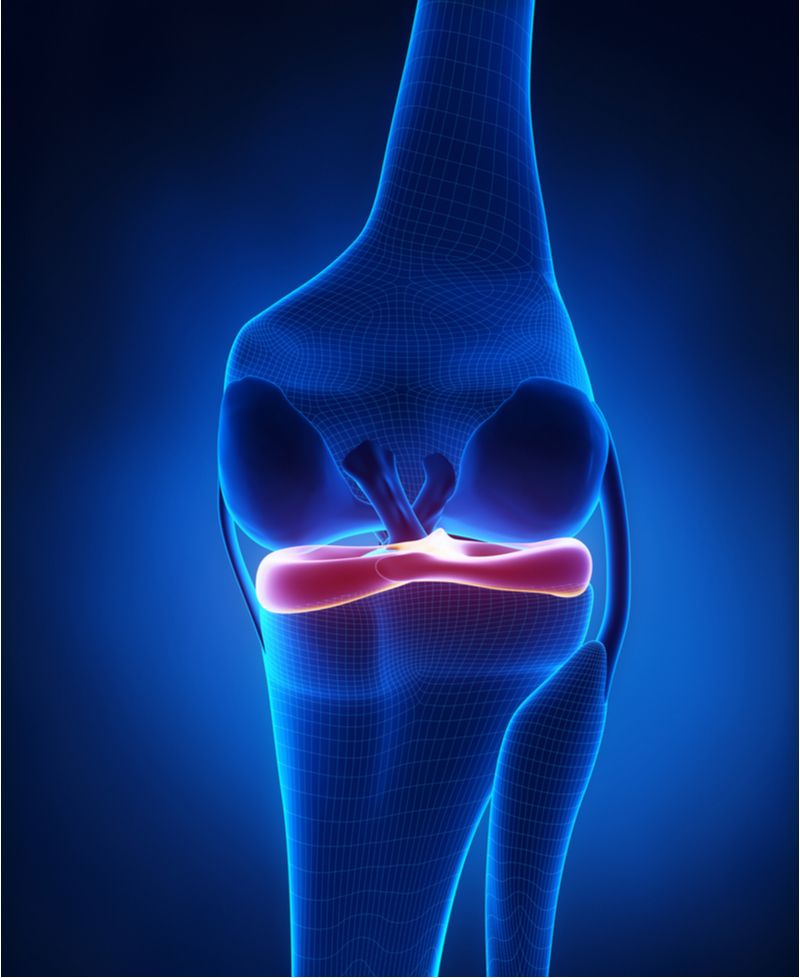
The fibrous structure of the C-shaped meniscus cartilage, which sits between the femur and tibia in the knee joint, is made up of the protein collagen, charged sugars called glycosaminoglycans, and water. The blood supply comes mainly from the peripheral synovium (the lining of the joint) with additional nutrition provided by the synovial fluid bathing the knee joint. This description also applies to the labrum of the shoulder—which keeps the humerus in place—and the labrum of the hip, which keeps the femur in the socket. As the tissue grows from infancy to adulthood, the cells in the fibrous tissue produce more collagen and more charged sugars. The tissue maintains its health throughout one’s lifetime. As is true of all tissues in the human body, unique stem cells within the tissue direct the healing process when an injury occurs.
Healthy meniscus tissue is resilient to tearing, but cannot survive the harsh pivoting that occurs in some football, rugby, and skiing maneuvers. When pinched hard between the femur and tibia, the meniscus collagen fibers separate. If the separation extends to the surface, a “tear” is seen on MRI as a white fluid signal traversing the tissue.
Healthy meniscus tissue, when torn due to an injury, can often be repaired with sutures and the stimulation of a fresh blood supply from the periphery. Nowadays, injections of growth factors from the patient’s own blood are introduced during the repair to augment healing.
If the meniscus tissue is unhealthy, however, the collagen fibers take on a disorganized appearance. The charged sugar matrix does not absorb water as it should, and the tissue becomes brittle as its ages. Under the microscope, the fibers look shriveled and thin. While aging alone may do this, it is far more likely that small injuries, leading to incomplete tears, build up over time. When injured and left untreated, the degenerative tissue within the meniscus expresses cytokines, also called chemotactic factors. Instead of promoting growth, these factors induce further decimation of the tissue, which the body tries unsuccessfully to degrade and remove. Many people walk around for decades with degenerative areas of their meniscus tissue, only discovering them when a final tear occurs—leading to pain, a diagnostic MRI, and surgery.
The question comes up: Can this degeneration be reversed before the complete tearing occurs, or can a degenerative meniscus be repaired or regenerated once it is found at surgery? In the past, the answer has usually been “no.” But now, thanks to an explosion in research, this is changing. The injection of growth factors, chemotactic agents, and collagen—all of which call the body’s stem-cell-derived self-repair cells to a site of injury—is becoming a form of preventive care, in addition to accelerating healing at the time of injury. One can envision a time when these degenerative areas of meniscus tissue (and shoulder, hip, and even chronic elbow tendonitis areas) will respond to a cocktail of growth factors. These factors will be designed to shift the tissue from disorganized, diseased collagen to healthy, organized collagen fibers—possibly preventing the final tearing that so often occurs in older patients.
An early example of this tissue replacement strategy was seen in collagen meniscus implants. Degenerative meniscus tears were cleaned out with surgical tools and a new collagen scaffold sewn into the gap, stimulating re-growth of the meniscus tissue. Years of follow-up data demonstrated that this system worked well and that the restoration of healthy collagen was indeed possible.
The next steps, once diseased meniscus tissue is identified, will be to intervene with injections of growth factor cocktails, along with new cells and injectable collagen, before surgery—possibly avoiding surgery altogether. For millions of patients, an ounce of biology may be worth a pound of sharpened steel.
